Now check out that Advertising Methods template embed again previously mentioned and consider how you would probably do it should you necessary to have These types of WHO processes in position.
Manufacturing and Handle operations are Obviously laid out in a penned form and GMP needs are adopted.
The eCFR is shown with paragraphs split and indented to Adhere to the hierarchy in the doc. That is an automated approach for user usefulness only and isn't intended to change company intent or existing codification.
Validated analytical methods are wanted for testing every batch, such as validation batches. The Agency would also assume the manufacturer to employ a validation protocol that includes an assessment and last report soon after several batches are accomplished, Though the sooner batches may well are actually distributed or Employed in the concluded drug product or service.
This contact form is just for Web site assistance or Internet site suggestions. When you've got queries or opinions relating to a printed document be sure to Make contact with the publishing company.
They have to adjust to EU GMP to obtain a manufacturing or import authorisation. They might make sure that they meet up with all their legal obligations by pursuing the EU GMP guidelines.
Of course. Typically, we feel that sampling in a typical drug manufacturing facility warehouse wouldn't stand for a threat into the container or closure or influence the integrity of the sample final results. But whether or not the act of collecting a sample while in the warehouse violates the CGMP requirement that containers "be opened, sampled, and sealed in the manner created to reduce contamination of their contents..." will depend upon the purported quality qualities of the fabric less click here than sample as well as the warehouse natural environment. For containers or closures purporting to be sterile or depyrogenated, sampling needs to be underneath conditions similar to the purported good quality of the fabric: a warehouse environment would not suffice (see 21 CFR 211.
Excellent manufacturing practice is recommended Together with the intention of safeguarding the overall health of people and patients in addition to generating good quality goods. In America, a food items or drug may be deemed "adulterated" if it has passed all of the technical specs checks but is discovered to get manufactured in the facility or condition which violates or won't adjust to present fantastic manufacturing guideline.
(b) The present superior manufacturing practice regulations With this chapter as they pertain to drug merchandise; in parts 600 by way of 680 of the chapter, because they pertain to prescription drugs that are also Organic merchandise for human use; and in part 1271 of the chapter, as They may be relevant to medications which are also human cells, tissues, and mobile and tissue-based mostly products (HCT/Ps) and that happen to be medicines (issue to overview under an software submitted less than area 505 of your act or beneath a Organic solution license software under portion 351 of the Public Wellbeing Service Act); health supplement and do not supersede the regulations In this particular part Except if the regulations explicitly deliver in any other case.
Sterile drug products and solutions ought to satisfy certain CGMP demands for personnel, buildings and amenities, components, manufacturing and controls, and screening, as suitable, to ensure merchandise sterility at the check here time of manufacture and through the entire merchandise’s shelf life.
When activated, PKG phosphorylates different concentrate on proteins, altering their functionality and contributing to cellular procedures which include easy muscle mass relaxation, ion channel regulation, and inhibition of platelet aggregation.
(1) There shall be a created assessment of stability centered at least on tests or examination from the drug products for compatibility on the substances, and dependant on advertising working experience Together with the drug merchandise to point that there's no degradation on the products for the traditional or expected duration of use.
What certain CGMP regulations could be practical to manufacturers of topical antiseptic drug merchandise?
(a) A technique whereby the oldest accredited inventory of the drug product is distributed first. Deviation from this need is permitted if these deviation is temporary and proper.
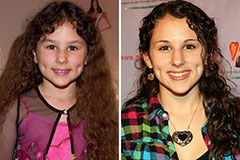 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Richard "Little Hercules" Sandrak Then & Now!
Richard "Little Hercules" Sandrak Then & Now! Kelly Le Brock Then & Now!
Kelly Le Brock Then & Now! Brooke Shields Then & Now!
Brooke Shields Then & Now! Lacey Chabert Then & Now!
Lacey Chabert Then & Now!